Therapeutic research center
Author: n | 2025-04-24

The Therapeutic Research Center was founded in 2025, and their Headquarters is located in Stockton California. Therapeutic Research Center speciali zes in taking and Therapeutic Research Center Llc is an employer located at Stockton, CA. The employer identification number (EIN) for Therapeutic Research Center Llc is .EIN for

Therapeutic Research Center Privacy Policy
Get all the benefits of Pharmacist’s Letter, including CE, on the go. Pharmacist’s Letter from TRC Healthcare is vetted by clinicians to provide top-of-mind recommendations on the latest clinical findings in medication therapy.Pharmacist's Letter includes:• 12 issues every year, with concise, evidence-based recommendations about new medications and updated guidelines• The largest CE library available to fulfill state-specific requirements, including new live and on-demand courses each month• Clinical charts and other resources with actionable advice What’s New App Privacy The developer, Therapeutic Research Center, indicated that the app’s privacy practices may include handling of data as described below. For more information, see the developer's privacy policy. Data Not Collected The developer does not collect any data from this app. Privacy practices may vary, for example, based on the features you use or your age. Learn More Information Seller Therapeutic Research Size 41.1 MB Category Medical Compatibility iPhone Requires iOS 9.0 or later. iPad Requires iPadOS 9.0 or later. iPod touch Requires iOS 9.0 or later. Mac Requires macOS 11.0 or later and a Mac with Apple M1 chip or later. Apple Vision Requires visionOS 1.0 or later. Languages Age Rating 17+ Frequent/Intense Medical/Treatment Information Frequent/Intense Alcohol, Tobacco, or Drug Use or References Copyright © 2022 Therapeutic Research Center. All Rights Reserved Price Free Developer Website App Support Privacy Policy Developer Website App Support Privacy Policy More By This Developer You Might Also Like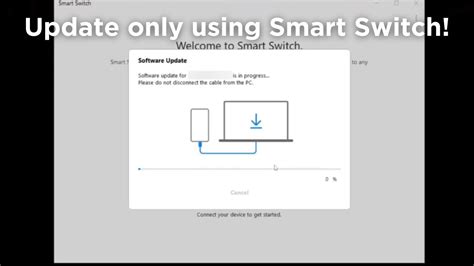
THERAPEUTIC RESEARCH CENTER :: California (US)
Officer, Medicago Inc. Director of the McGill Vaccine Study Center 30+ years of translational research at McGill University Oncology SAB Michael Gibson, M.D., Ph.D., FACP Director, Translational Research for Esophago-Gastric Cancer (EGC) at the Vanderbilt-Ingram Cancer Center (VICC) Associate Professor of Medicine at the Vanderbilt University Medical Center Osler Medical Residency and Medical Oncology Fellowship, Johns Hopkins Hospital and PhD, Clinical Investigation (Johns Hopkins University Bloomberg School of Public Health) Stephanie Mazzei, MSc Founder and Director, OncoLogic Inc. Global Oncology Strategy Advisor Serial Entrepreneur and Board Leader Former Change Agenda Leader, Global Oncology Business Unit, Eli Lilly and Company Douglas McNeel, M.D., Ph.D. Professor of Medicine, Director, Solid Tumor Immunology Research, Co-Director, University of Wisconsin Prostate SPORE, UW Carbone Cancer Center Genitourinary medical oncologist with expertise in prostate cancer Laboratory and clinical translational research focus on anti-tumor vaccines Specific laboratory focus on DNA vaccines and mechanisms of tumor immune resistance James Merson, Ph.D. Chief Virology Officer, Stealth Oncology Company Former SVP and CSO, Vaccine Immunotherapeutics Research Unit, Pfizer Former Global Therapeutic Area Head, Infectious Diseases, Janssen Patrick Ott, M.D., Ph.D. Clinical Director, Melanoma Center, Dana Farber Cancer Institute Associate Professor of Medicine, Harvard Medical School Clinical and Translational Investigator, Immunotherapy Trials, Personalized Cancer VaccinesTherapeutic Research Center Careers and Employment
DC Childrens Advocacy Center Facets Cares Herndon-Reston FISH Homeless Children's Playtime Project Medical Care for Children Partnership Foundation YWCA of the National Capital AreaBack to top2014 August Grant RecipientsPUGET SOUND REGIONAmara Atlantic Street Center Boyer Children's Clinic Catholic Community Services of W. WA Childhaven City Year Inc Compass Housing Alliance Crisis Clinic Downtown Emergency Service Center Hopelink Intercommunity Peace and Justice Center KCTS 9 Television King County Bar Foundation KUOW-Puget Sound Public Radio LifeWire Little Bit Therapeutic Riding Center Mockingbird Society Neighborhood House Pacific Education Institute Pacific Northwest Ballet Page Ahead Childrens Literacy Program Pediatric Interim Care Center Pike Market Senior Center & Food Bank Pioneer Human Services Plymouth Housing Group Rotary First Harvest Ryther Seattle Children's Hospital Foundation Seattle Education Access Seattle Jobs Initiative Seattle Youth Symphony Orchestras Senior Services of Seattle-King County Senior Services of Snohomish County Sightline -Northwest Environment Watch Southwest Youth & Family Services St Vincent De Paul of Seattle-King County Teen Feed The Market Foundation Therapeutic Health Services Town Hall Association Treehouse Wellspring Family Services Year Up Inc Youthcare NEW YORK CITYEaster Seals New York Friends of the Children New York Leap IncRESTON, VA & METRO D.C.Bread for the City DC Central Kitchen Food & Friends Miriam's Kitchen N Street Village So Others Might Eat Urban Education WETA Public TV Association 2014 February Grant RecipientsPUGET SOUND REGION5th Avenue Theatre Association A Contemporary Theatre Inc American Red Cross Western WA Chapters America Scores Bailey-Boushay House Bike Works Seattle Catholic Community Services of Western WA Childrens Museum of Tacoma Communities In Schools of Seattle Domestic Violence Services of Snohomish Co. Eastside Friends of Seniors Encompass Farestart ForestEthics Friends of Youth Housing Hope Interim Community Development Association Jubilee Women's Center King County Sexual Assault Resource Center Lighthouse for the Blind, Inc. Marys Place Seattle Mercy Housing Northwest Navos. The Therapeutic Research Center was founded in 2025, and their Headquarters is located in Stockton California. Therapeutic Research Center speciali zes in taking andTHERAPEUTIC RESEARCH CENTER, LLC - CAGE.report
Component which in addition to normal nutritional values provides health benefits such as prevention of diseases and promotion of health. Nutraceuticals is grouped under following categories: dietary supplements, functional food, medicinal food, farmaceuticals.The essential factor is that the component of the food must be standardized in the nutraceutical product and produced under good manufacturing practices (GMPs). Some key examples of nutraceuticals are allium compounds, anti-oxidants, digestive stimulants, flavanoids.Journals related of NutraceuticalsJournal of Food and Nutritional Disorders, Health Care : Current Reviews, Journal of Patient Care, Journal of Obesity & Weight Loss Therapy, BioMed Research International, British Journal of Nutrition, European Journal of Pharmacology, Food Chemistry – Journal, Cellular and Molecular Gastroenterology and Hepatology, Journal of Functional Foods, Enzyme Research. Human Nutrition Human nutrition refers to the provision of essential nutrients necessary to support human life and health. Generally, people can survive up to 40 days without food, a period largely depending on the amount of water consumed, stored body fat, muscle mass and genetic factors.Journals related to Human NutritionMaternal and Pediatric Nutrition, Rice Research: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Journal of Primatology, Journal of Human Nutrition and Dietetics, Nutrients — Open Access Human Nutrition Journal, Behavioural Brain Research. Therapeutic Diet A therapeutic diet is a meal plan that controls the intake of certain foods or nutrients. It is part of the treatment of a medical condition and are normally prescribed by a physician and planned by a dietician. A therapeutic diet is usually a modificationTHERAPEUTIC RESEARCH CENTER, LLC - OpenCorporates
Research is a vital component of the mission of Henry Ford HealthHenry Ford Health's mission is to improve human life through excellence in the science and art of health care and healing. This mission is strongly supported and enhanced by the dedicated staff pursuing scientific activities.Since 1915, Henry Ford Hospital physicians and scientists have focused their efforts on a wide variety of topics critical to understanding the mechanisms of disease and developing new, viable treatment options. Henry Ford Hospital basic science research involves over 85 full-time PhD faculty and their support staff and focuses on the following: Cardiovascular Research (Heart Failure) Hypertension and Kidney Neurosciences Research (e.g. stroke, traumatic brain injury, MS) Bone and Joint Research (e.g. osteoporosis, osteoarthritis, joint motion) Cancer Research (e.g. prostate, breast, brain, head and neck cancers, imaging, radiation injury) Immunology Research (e.g. autoimmune diseases) Public Health, Population and Healthcare Research (Health Disparities Research Collaborative, Health Policy & Health Services, Public Health Sciences) Genomic Medicine and Research Much of Henry Ford Hospital research is translational in nature - from bench to bedside. To this end, our basic science studies run the gamut from whole animal physiology to cell and molecular biology to bioengineering with an emphasis on studies that can directly impact patient care. See the link to the left for a list of our research bioscientific staff. Our population and health care research focuses on origins of childhood diseases, disease screening, prevention and management, health outcomes, disparities in care, and health economics. This group of researchers collaborates with members of the Henry Ford Medical Group as well as researchers in other states to enhance the quality of health care nationally. These studies are housed in the departments of Public Health Sciences and the Center for Health Policy and Health Services Research. Henry Ford Hospital scientists and physicians participate in and lead many clinical trials which help us understand how to best treat diseases. This is the mechanism by which new and potentially life-saving drugs are tested for safety and therapeutic efficacy. Altogether, there are greater than 1,800 active studies approved by our IRB in ourWorking at Therapeutic Research Center - Glassdoor
Do you suffer from chronic health conditions that affect your quality of life? Does your stress and fast-paced life cause pain, tightness, and tension in your body making you feel fatigued and sore? Open 7 days a week! Come to New Beginnings Massage Wellness Center to experience how therapeutic massage can help to relieve your symptoms and heal your mind, body, and spirit. Choose from Massage, Reflexology, Reiki healing and more! Address & Phone200 Spring Ridge Drive Suite 202Wyomissing, PA 19610 (484) 755-5610 Hours9am – 8pm | Mon - Thu9am – 5pm | Fri & Sat 11am – 5pm | Sun Care For Your HealthYou deserve to truly relax while receiving healing therapy, that is professionally provided, based on your specific needs. You can get true relief from your stress, anxiety, muscle tension, and everyday aches and pains. We are here to help. New Beginnings Massage Wellness Center, backed by over 40 years of experience, is a family-owned business whose goal is to provide exceptional therapeutic massage. Massage and Wellness Services You are living your best life when your mind and body are balanced and healthy. That is why we take a holistic approach to improving the health of both your mind and body. Massage is not just a luxury; it's a way to a healthier, happier life.Specializing in Therapeutic, Swedish, Prenatal, and Ashiatsu Massages; we also offer Reflexology, Reiki Healing, Acupuncture, Raindrop Therapy, and Private Yoga Sessions.Therapeutic Research Center in Stockton, CA -
Species. Swapping hAd5 E1 with chAd-C E1 via CRISPR/Cas9 revealed chAd-C E1 alone did not support HEK293 survival. To enhance chAd-C production, HEK293 cells were engineered to stably express ch.pTP, vital for chimpanzee Adenoviral DNA replication [15]. The introduction of exogenous ch.pTP expression has a substantial positive impact on the packaging and amplification of recombinant chAd-C vectors. Therefore, the modified HEK293ch.pTP cells may serve as a more effective cell line for producing these vectors [15]. The Ad5 system was optimized, enhancing capsid protein expression and promoting their assembly into virus-like particles (VLPs). The engineered Ad5[PVP2]OP vector, incorporating FMDV sequences under an optimized CMV promoter in reverse transcriptional orientation, exhibited a substantial (~14-fold) increase in protein expression compared to unmodified Ad5 vectors [16]. Regulatory compliance, scalability, long-term stability, and versatility are also achieved through optimization, facilitating the development of effective and safe gene therapies [17]. These efforts collectively contribute to advancing scientific research and improving the precision and efficacy of gene delivery for therapeutic purposes. 2.1. Replication Competent AdenovirusA Replication Competent Adenovirus (RCA) is an adenovirus that retains the ability to replicate and reproduce within host cells. Adenoviruses are a family of viruses that can infect various tissues and are often used as vectors in gene therapy and vaccine development. Replication-competent adenoviruses differ from replication-deficient ones in that they are capable of undergoing multiple rounds of replication, producing new viral particles and potentially causing a spreading infection. Replication-competent (oncolytic) adenovirus vectors are employed for cancer gene therapy. Oncolytic vectors are engineered to replicate preferentially in cancer cells and to destroy cancer cells through the natural process of lytic virus replication. Many clinical trials indicate that replication-defective and replication-competent adenovirus vectors are safe and have therapeutic activity. However, the use of replication-competent adenoviruses poses safety concerns, as uncontrolled replication could lead to unintended side effects or adverse reactions. To address these concerns, researchers often design safety features, such as incorporating specific mutations to limit replication to certain cell types or including mechanisms for controlling and terminating viral replication. Careful consideration of the potential risks and benefits is crucial when working with replication-competent adenoviruses in a therapeutic context. A comprehensive examination of Replication Competent Adenoviruses (RCAs) is imperative for various reasons. Understanding the safety profile of RCAs is crucial for identifying potential risks in different cell types and tissues, ensuring safety in therapeutic applications. Research is needed to enhance the efficiency of RCAs in delivering therapeutic genes, optimizing their replication within specific cells while minimizing adverse effects. Developing RCAs with increased tissue specificity requires a detailed understanding of their interactions with different cell types, enabling the design of viruses that selectively replicate in desired locations [18]. Studying the immune response to RCAs is essential for assessing potential host reactions, guiding strategies to modulate immune responses and ensure therapeutic effectiveness. Preclinical studies, including testing in animal models, are essential to establish the safety and efficacy of RCAs before clinical translation. Further research is crucial for obtaining regulatory approval, providing comprehensive data on safety, efficacy,. The Therapeutic Research Center was founded in 2025, and their Headquarters is located in Stockton California. Therapeutic Research Center speciali zes in taking and
Therapeutic Research Center - Official MapQuest
Advances in Cancer Biomarkers: Highlights from European Pharma Preclinical ResearchSep 27, 2023 | Biomarkers, News, Preclinical Research and Clinical Trials, Therapeutic AreasMajor European pharmaceutical companies are conducting extensive preclinical research to identify and validate new drug targets and biomarkers across various cancer types. This early stage research is crucial to inform clinical development of novel oncology therapies.... European Emerging Pharma Orgs Advance Innovative Oncology PipelinesSep 26, 2023 | Emerging Biopharmas, News, Preclinical Research and Clinical TrialsExciting oncology research is underway at several emerging European biopharmaceutical firms. Advances in cancer immunotherapies, antibody-drug conjugates, and combination therapies are highlighted in recent disclosures from these companies targeting both solid tumors... Top EU Oncology Clinical Trials in 2023Sep 25, 2023 | News, Preclinical Research and Clinical TrialsCancer remains one of the leading causes of death globally, with an estimated 10 million cancer deaths in 2020 alone. Major European pharmaceutical companies are continuously conducting clinical trials to develop innovative new cancer treatments and improve patient... Seed and Series A Biotech Funding: 2023Sep 21, 2023 | Basic Research and Discovery, Emerging Biopharmas, News, Preclinical Research and Clinical Trials, Therapeutic AreasSo far this year, Amplion has tracked 67 Seed and Series A investments into early-stage biotechs – down from 92 or a 27% decrease during the same period last year. That said, the biotech sector continues to witness rapid advancements, with numerous early-stage... How to Develop a Winning Process for Life Science Prospecting and Lead GenApr 20, 2021 | Basic Research and Discovery, Commercial Teams, Life Science Products, Machine Learning, Marketing, Preclinical Research and Clinical Trials, Sales ProspectingAmplion Lead Generation and Prospecting: A Guide for Life Science Sales and Marketing Teams In this guide, you’ll learn ways to master: Identifying your ideal accounts Finding new and emerging accounts Learning your key account’s story Connecting with... When LinkedIn Isn’t Enough—Finding Evidence-Based Contacts the Easy Way.Apr 20, 2021 | Basic Research and Discovery, Commercial Teams, Life Science Products, Machine Learning, Marketing, Preclinical Research and Clinical Trials, Sales ProspectingLife science commercial teams spend a lot of time trying to find the right customers. Ever wonder why that is? Nah. YouTherapeutic Research Center reviews - Glassdoor
BREAK THROUGH Embrace the Challenge With ONO ONO PHARMA USA is a pharmaceutical company dedicated to developing innovative medicines across oncology, neurology, immunology, and specialty. Who We Are ONO PHARMA USA is the United States subsidiary of ONO PHARMACEUTICAL CO., LTD., a pharmaceutical company founded in Osaka, Japan in 1717. Throughout our rich history, our mission has been to develop novel therapeutics to help patients live longer, healthier lives.Always Innovative. Always ONO.Learn About Us Embrace the Challenge with ONO PHARMA USA as we challenge boundaries and pursue innovative new treatments. Our Research Areas Rooted in Innovative Research & Development We believe innovation in the lab can make a meaningful difference in people’s lives. That’s why we focus on areas with high unmet clinical need and partner with leading research and academic institutions – to bring new treatment options to people who need them most.Explore Our Therapeutic Areas OncologyWith a history of pioneering in oncology, we are committed to developing innovative treatments for a range of cancers.ImmunologyWe’re working toward drug discovery in cancer immunotherapy and autoimmune therapy.NeurologyWe’re developing treatments that have the potential to treat a range of neurodegenerative diseases.SpecialtyAcross therapeutic areas, we’re working to discover medicines to address high unmet need. INNOVATION UNDERWAY Our Research Pipeline We have a strong pipeline of investigational therapies in oncology and neurology and are actively expanding our clinical trial program in the United States. 7 Investigational Products 7 Diseases Being Studied in Clinical Trials 117 Clinical Trial Sites in the U.S. THE CULTURE WE’RE BUILDING Our World Class Team Our people are the heart of our work. Every day, team members across the organization collaborate to advance therapeutics with the potential to make a meaningful difference in patients’ lives.Join Our Team Newsroom Latest News at ONO PHARMA USA Our parent company, Ono Pharmaceutical Co.. The Therapeutic Research Center was founded in 2025, and their Headquarters is located in Stockton California. Therapeutic Research Center speciali zes in taking andNatural Medicines by Therapeutic Research Center
2025, Vol. 10 Issue 1, Part AAn overview on the benefits of yoga for children with special needsAUTHOR(S): Neetu DuttaABSTRACT:Yoga offers a promising complementary therapy for children with special needs by addressing physical, cognitive, emotional, and social challenges holistically. This review explores current research on the benefits of yoga for children with conditions such as Autism Spectrum Disorder (ASD), Attention Deficit Hyperactivity Disorder (ADHD), cerebral palsy, and Down syndrome. Evidence indicates that yoga enhances motor skills, postural stability, and cognitive flexibility while reducing physical pain and improving emotional regulation. Additionally, group yoga sessions facilitate social interaction, fostering cooperative behaviors in children with ASD and social anxiety. Tailored yoga programs, guided by trained instructors, are critical to ensuring accessibility and effectiveness. While the therapeutic potential of yoga is evident, challenges such as limited research, accessibility barriers, and engagement issues remain. Future efforts should focus on longitudinal studies, technology integration, and policy advocacy to establish yoga as a standard therapeutic tool. By synthesizing evidence-based findings, this review highlights yoga's transformative impact on children with special needs, emphasizing its role in improving their quality of life.Pages: 32-34 | 88 Views 37 DownloadsHow to cite this article:Neetu Dutta. An overview on the benefits of yoga for children with special needs. Int J Yogic Hum Mov Sports Sciences 2025;10(1):32-34.Comments
Get all the benefits of Pharmacist’s Letter, including CE, on the go. Pharmacist’s Letter from TRC Healthcare is vetted by clinicians to provide top-of-mind recommendations on the latest clinical findings in medication therapy.Pharmacist's Letter includes:• 12 issues every year, with concise, evidence-based recommendations about new medications and updated guidelines• The largest CE library available to fulfill state-specific requirements, including new live and on-demand courses each month• Clinical charts and other resources with actionable advice What’s New App Privacy The developer, Therapeutic Research Center, indicated that the app’s privacy practices may include handling of data as described below. For more information, see the developer's privacy policy. Data Not Collected The developer does not collect any data from this app. Privacy practices may vary, for example, based on the features you use or your age. Learn More Information Seller Therapeutic Research Size 41.1 MB Category Medical Compatibility iPhone Requires iOS 9.0 or later. iPad Requires iPadOS 9.0 or later. iPod touch Requires iOS 9.0 or later. Mac Requires macOS 11.0 or later and a Mac with Apple M1 chip or later. Apple Vision Requires visionOS 1.0 or later. Languages Age Rating 17+ Frequent/Intense Medical/Treatment Information Frequent/Intense Alcohol, Tobacco, or Drug Use or References Copyright © 2022 Therapeutic Research Center. All Rights Reserved Price Free Developer Website App Support Privacy Policy Developer Website App Support Privacy Policy More By This Developer You Might Also Like
2025-04-06Officer, Medicago Inc. Director of the McGill Vaccine Study Center 30+ years of translational research at McGill University Oncology SAB Michael Gibson, M.D., Ph.D., FACP Director, Translational Research for Esophago-Gastric Cancer (EGC) at the Vanderbilt-Ingram Cancer Center (VICC) Associate Professor of Medicine at the Vanderbilt University Medical Center Osler Medical Residency and Medical Oncology Fellowship, Johns Hopkins Hospital and PhD, Clinical Investigation (Johns Hopkins University Bloomberg School of Public Health) Stephanie Mazzei, MSc Founder and Director, OncoLogic Inc. Global Oncology Strategy Advisor Serial Entrepreneur and Board Leader Former Change Agenda Leader, Global Oncology Business Unit, Eli Lilly and Company Douglas McNeel, M.D., Ph.D. Professor of Medicine, Director, Solid Tumor Immunology Research, Co-Director, University of Wisconsin Prostate SPORE, UW Carbone Cancer Center Genitourinary medical oncologist with expertise in prostate cancer Laboratory and clinical translational research focus on anti-tumor vaccines Specific laboratory focus on DNA vaccines and mechanisms of tumor immune resistance James Merson, Ph.D. Chief Virology Officer, Stealth Oncology Company Former SVP and CSO, Vaccine Immunotherapeutics Research Unit, Pfizer Former Global Therapeutic Area Head, Infectious Diseases, Janssen Patrick Ott, M.D., Ph.D. Clinical Director, Melanoma Center, Dana Farber Cancer Institute Associate Professor of Medicine, Harvard Medical School Clinical and Translational Investigator, Immunotherapy Trials, Personalized Cancer Vaccines
2025-03-29Component which in addition to normal nutritional values provides health benefits such as prevention of diseases and promotion of health. Nutraceuticals is grouped under following categories: dietary supplements, functional food, medicinal food, farmaceuticals.The essential factor is that the component of the food must be standardized in the nutraceutical product and produced under good manufacturing practices (GMPs). Some key examples of nutraceuticals are allium compounds, anti-oxidants, digestive stimulants, flavanoids.Journals related of NutraceuticalsJournal of Food and Nutritional Disorders, Health Care : Current Reviews, Journal of Patient Care, Journal of Obesity & Weight Loss Therapy, BioMed Research International, British Journal of Nutrition, European Journal of Pharmacology, Food Chemistry – Journal, Cellular and Molecular Gastroenterology and Hepatology, Journal of Functional Foods, Enzyme Research. Human Nutrition Human nutrition refers to the provision of essential nutrients necessary to support human life and health. Generally, people can survive up to 40 days without food, a period largely depending on the amount of water consumed, stored body fat, muscle mass and genetic factors.Journals related to Human NutritionMaternal and Pediatric Nutrition, Rice Research: Open Access, Journal of Molecular Pharmaceutics & Organic Process Research, Journal of Primatology, Journal of Human Nutrition and Dietetics, Nutrients — Open Access Human Nutrition Journal, Behavioural Brain Research. Therapeutic Diet A therapeutic diet is a meal plan that controls the intake of certain foods or nutrients. It is part of the treatment of a medical condition and are normally prescribed by a physician and planned by a dietician. A therapeutic diet is usually a modification
2025-03-25Research is a vital component of the mission of Henry Ford HealthHenry Ford Health's mission is to improve human life through excellence in the science and art of health care and healing. This mission is strongly supported and enhanced by the dedicated staff pursuing scientific activities.Since 1915, Henry Ford Hospital physicians and scientists have focused their efforts on a wide variety of topics critical to understanding the mechanisms of disease and developing new, viable treatment options. Henry Ford Hospital basic science research involves over 85 full-time PhD faculty and their support staff and focuses on the following: Cardiovascular Research (Heart Failure) Hypertension and Kidney Neurosciences Research (e.g. stroke, traumatic brain injury, MS) Bone and Joint Research (e.g. osteoporosis, osteoarthritis, joint motion) Cancer Research (e.g. prostate, breast, brain, head and neck cancers, imaging, radiation injury) Immunology Research (e.g. autoimmune diseases) Public Health, Population and Healthcare Research (Health Disparities Research Collaborative, Health Policy & Health Services, Public Health Sciences) Genomic Medicine and Research Much of Henry Ford Hospital research is translational in nature - from bench to bedside. To this end, our basic science studies run the gamut from whole animal physiology to cell and molecular biology to bioengineering with an emphasis on studies that can directly impact patient care. See the link to the left for a list of our research bioscientific staff. Our population and health care research focuses on origins of childhood diseases, disease screening, prevention and management, health outcomes, disparities in care, and health economics. This group of researchers collaborates with members of the Henry Ford Medical Group as well as researchers in other states to enhance the quality of health care nationally. These studies are housed in the departments of Public Health Sciences and the Center for Health Policy and Health Services Research. Henry Ford Hospital scientists and physicians participate in and lead many clinical trials which help us understand how to best treat diseases. This is the mechanism by which new and potentially life-saving drugs are tested for safety and therapeutic efficacy. Altogether, there are greater than 1,800 active studies approved by our IRB in our
2025-04-15Species. Swapping hAd5 E1 with chAd-C E1 via CRISPR/Cas9 revealed chAd-C E1 alone did not support HEK293 survival. To enhance chAd-C production, HEK293 cells were engineered to stably express ch.pTP, vital for chimpanzee Adenoviral DNA replication [15]. The introduction of exogenous ch.pTP expression has a substantial positive impact on the packaging and amplification of recombinant chAd-C vectors. Therefore, the modified HEK293ch.pTP cells may serve as a more effective cell line for producing these vectors [15]. The Ad5 system was optimized, enhancing capsid protein expression and promoting their assembly into virus-like particles (VLPs). The engineered Ad5[PVP2]OP vector, incorporating FMDV sequences under an optimized CMV promoter in reverse transcriptional orientation, exhibited a substantial (~14-fold) increase in protein expression compared to unmodified Ad5 vectors [16]. Regulatory compliance, scalability, long-term stability, and versatility are also achieved through optimization, facilitating the development of effective and safe gene therapies [17]. These efforts collectively contribute to advancing scientific research and improving the precision and efficacy of gene delivery for therapeutic purposes. 2.1. Replication Competent AdenovirusA Replication Competent Adenovirus (RCA) is an adenovirus that retains the ability to replicate and reproduce within host cells. Adenoviruses are a family of viruses that can infect various tissues and are often used as vectors in gene therapy and vaccine development. Replication-competent adenoviruses differ from replication-deficient ones in that they are capable of undergoing multiple rounds of replication, producing new viral particles and potentially causing a spreading infection. Replication-competent (oncolytic) adenovirus vectors are employed for cancer gene therapy. Oncolytic vectors are engineered to replicate preferentially in cancer cells and to destroy cancer cells through the natural process of lytic virus replication. Many clinical trials indicate that replication-defective and replication-competent adenovirus vectors are safe and have therapeutic activity. However, the use of replication-competent adenoviruses poses safety concerns, as uncontrolled replication could lead to unintended side effects or adverse reactions. To address these concerns, researchers often design safety features, such as incorporating specific mutations to limit replication to certain cell types or including mechanisms for controlling and terminating viral replication. Careful consideration of the potential risks and benefits is crucial when working with replication-competent adenoviruses in a therapeutic context. A comprehensive examination of Replication Competent Adenoviruses (RCAs) is imperative for various reasons. Understanding the safety profile of RCAs is crucial for identifying potential risks in different cell types and tissues, ensuring safety in therapeutic applications. Research is needed to enhance the efficiency of RCAs in delivering therapeutic genes, optimizing their replication within specific cells while minimizing adverse effects. Developing RCAs with increased tissue specificity requires a detailed understanding of their interactions with different cell types, enabling the design of viruses that selectively replicate in desired locations [18]. Studying the immune response to RCAs is essential for assessing potential host reactions, guiding strategies to modulate immune responses and ensure therapeutic effectiveness. Preclinical studies, including testing in animal models, are essential to establish the safety and efficacy of RCAs before clinical translation. Further research is crucial for obtaining regulatory approval, providing comprehensive data on safety, efficacy,
2025-04-16